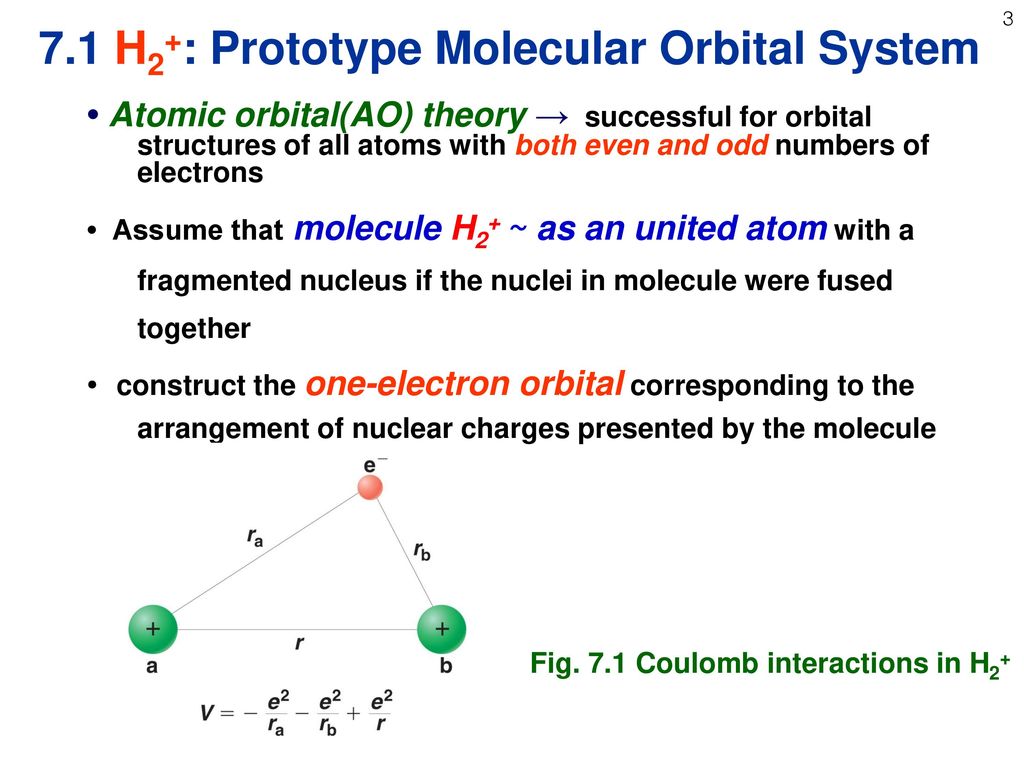
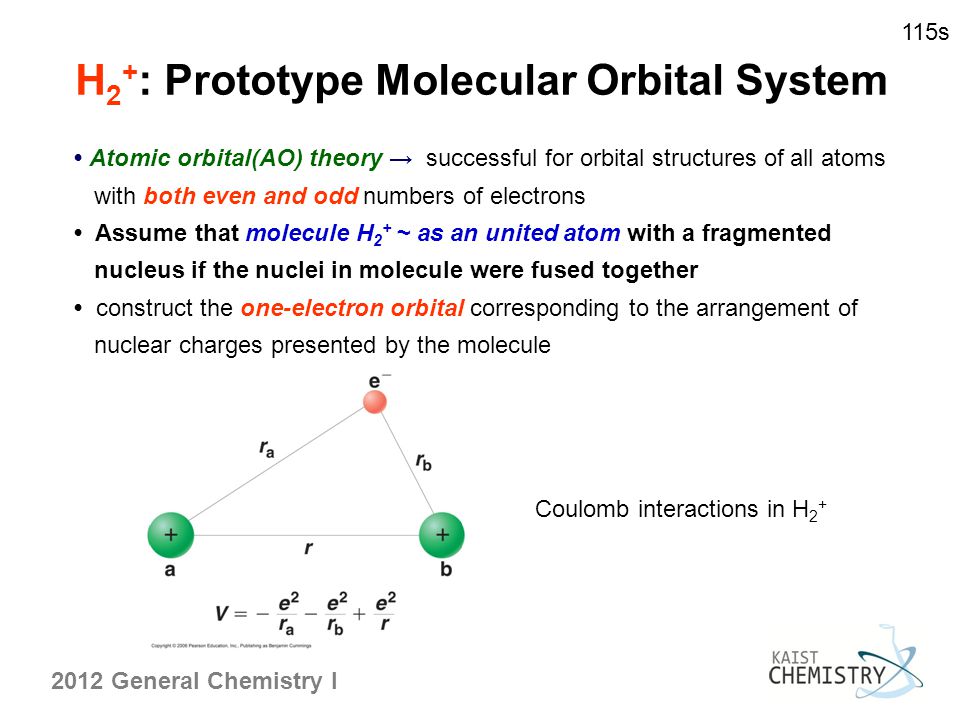
MO Theory Assumption: The 2 or more nuclei are placed at equilibrium distance and electrons are added to molecular orbitals in much the same way as electrons are added to atomic orbitals in atoms The 3 principles (Pauli exclusion principle, aufbau principle and Hunds rule) also apply For a given molecule, a wave equation can be written. Molecular Orbital Theory The goal of molecular orbital theory is to describe molecules in a similar way to how we describe atoms, that is, in terms of orbitals, orbital diagrams, and electron configurations. For example, to give you a glimpse at where we are headed, the following are orbital diagrams for O. Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energeticallyfavored configuration. The Hydrogen Molecule Ion H 2 The LCAO method adopts an especially simple form for homonuclear diatomic molecules, i. molecules that consist of two identical atoms, e. It is recommendable to begin with the most simple among those systems, the hydrogen molecule ion H 2. Using Symmetry: Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. The Molecular Orbital (MO) approach is an alternate approach that proposes that electrons belong to the entire molecule. Except for some very simple cases, a correct application of MO to even simple molecules such as water does require considerable knowledge of the techniques of quantum mechanics. Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, and shape. Molecular Orbital (MO) Theory Lecture 2 Simple Molecular Orbitals Sigma and Pi Bonds in Molecules bond order (number of bonding electrons) (number of antibonding elect rons) 2 amount of bonding 1sa hydrogen molecule H2 LUMO HOMO 1sa 1sb bonding MO potential energy higher, less stable lower, more stable LUMO lowest unoccupied molecular orbital HOMO. The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to for other diatomic molecules, but involving more than the 1 s atomic orbitals. Molecular Orbital Theory is primarily used to explain the bonding in molecules that cannot be explained by Valence Bond Theory. These are molecules that generally involve some form of resonance. Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wave functions. A molecular orbital can give information about the electron configuration of a molecule. The electron configuration is the most likely position, and the energy of one (or one pair of) electron(s). The electron configuration is the most likely position, and the energy of one (or one pair of) electron(s). Use molecular orbital theory to predict whether or not each of the following molecules or ions should exist in a relatively stable form. The Hydrogen molecule We are now in a position to discuss the electronic structure of the simplest molecule: Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the MO theory predicts a binding energy of 5. Since molecular oxygen contains two electrons in an antibonding orbital, it might be possible to make the molecule more stable by removing one of these electrons, thus increasing the ratio of bonding to antibonding electrons in the molecule. Topics: Molecular orbital theory I. Bonding and antibonding orbitals II. Homonuclear diatomic molecules Molecule is more stable than the individual atoms. Molecular wave function (molecular orbital) with a nodal plane through the axis. Molecular orbital theory doesnt deal with resonance, but it makes resonance more understandable. Whenever you can draw two or more Lewis structures for a molecule, the actual structure is none of the structures but is a resonance hybrid of them all. Valence bond theory is able to explain many aspects of bonding, but not all. To complement this theory, we use another called the molecular orbital (MO) theory. Molecular orbital theory is a more sophisticated model for understanding the nature of chemical bonding. Each orbital accommodates two electrons, and the two electrons in HH filles the s 1s molecular orbital (MO). Obviously, as a result of the formation of H 2 molecule, the energy of the system is lowered and become more stable. According to MO theory, the two atomic 2p z orbitals combine to form two pi () molecular orbitals, one a lowenergy bonding orbital and one a highenergy star ( ) antibonding molecular orbital. In molecular orbital theory, we postulate that the combination of atomic orbitals on different atoms forms molecular orbitals (MOs), so that electrons in them belong to the molecule as a whole. Introduction to Molecular Orbital Theory. This is the bondingmolecular orbital In the water molecule the highest occupied orbital, (1b 1) is nonbonding and highly localized on the oxygen atom, similar to the nonbonding orbitals of hydrogen fluoride. Molecular Orbital Theory For example, when two hydrogen atoms bond, for the H2 molecule. (b) The shapes of the molecular orbitals are obtained the bonding molecular orbital of the HF molecule. Note the greater electron density close to the fluorine atom. 45: The resonance structures 09 Bonding MO Theory 6. Hybridization and Molecular Orbital (MO) Theory Chapter 10 Molecular orbital theory (MO) a molecule is formed by the overlap of atomic orbitals to form molecular orbitals, electrons are then distributed into MOs. A molecule is a collection of The electronic and. According to the molecular orbital theory, in a supposed He2 molecule, both the bonding and the antibonding orbitals will have 2 electrons each. And so, the bond order (half the difference in the numbers of bonding and antibonding electrons) for such a diatomic entity is zero. Molecular orbital (MO) theory has the potential to be more quantitative. With it we can also get a picture of where the electrons are in the molecule, as shown in the image at the right. This can help us understand patterns of bonding and reactivity that are otherwise difficult to explain. Testin g qualitative MO theory prediction of Bond Order with experiment for homonuclear diatomics made from elements in the 1 st row of the Periodic Table (using the Molecular Orbital Aufbau principle). 13 Molecular Orbital Theory BH 3 B H H H z y x The BH 3 molecule exists in the gas phase, but dimerizes to B 2H6 (which we will look at a bit later) 2 BH 3 B2H6 The BH 3 molecule is trigonal planar and we will make the C 3 principal axis of symmetry the z axis, with the x and y axes in the plane Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO VIII. OverviewDefinitions So far, we have been visualizing covalent bonds (molecular orbitals) as regions where electrons are being shared between atomic orbitals (that may or may not be hybridized). Molecular Orbital Theory Electrons go into the lowest energy orbital available to form lowest potential energy for the molecule. The maximum number of electrons in each molecular orbital is two. (Pauli exclusion principle) One electron goes into orbitals of equal energy, with. An MO energy level diagram for H2 is (the molecular orbitals are not yet labeled, nor are the arrows representing electrons drawn yet for H2): 1s of one H AO of H 1s of 2nd H MOs of H2 AO of H a. Label the MOs (one with, the other with ) in the above energy level diagram. A question in my general chemistry textbook gives me a list of formulas for molecules and ions. It then asks me to determine, using molecular orbital theory, whether or. H 1s antibonding molecular orbital In chemical bonding theory, an antibonding orbital is a type of molecular orbital (MO) that weakens the bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. The highest occupied molecular orbital (HOMO), 1b 1, is predominantly p z 2 in character with no contribution from the hydrogen 1s orbital and mainly contributes to the lone pair effects. The 2 a 1, 1 b 2 and 3 a 1 all contribute to the OH bonds. Each orbital accommodates two electrons, and the two electrons in \(\ceHH\) fill the s 1s molecular orbital (MO). Obviously, as a result of the formation of the \(\ceH2\) molecule, the energy of the system is lowered and becomes more stable. MO Theory 1 Molecular Orbital (MO) Theory Lewis dot, VESPR Valence Bond (VB) theories all do a good job at predicting the shapes and bonding in covalent molecules. Chemists however sometimes require another theory of bonding that explains phenomenon For the ion H2: a) Draw the molecular orbital diagram. Molecular Orbital Theory Build Hydrogen H2 Duration: How to Determine if a Molecule is Polar or Not. A more general, but slightly more complicated approach is the Molecular Orbital Theory. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. 7 Molecular Orbitals 369 The lowerenergy MO of H 2 concentrates electron density between the two hydrogen nuclei and is called the bonding molecular orbital. This sausageshaped MO results from summing the two atomic orbitals so that the atomic or Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at particular points around the molecule. To produce the set of orbitals for a molecule, we add together the valence atomic wavefunctions for. Molecular Orbital Theory in analogy to the term atomic orbital. To describe H2, we can say that the two electrons occupy molecular orbitals like that of H2, one with a spin and one with fJ spin. The effect of interelectronic repulsion is to decrease Description of the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this video are: bonding MO's, antibonding MO's, and bond order. Problem: Draw MO energy diagrams for the molecular ions H2 and H2. Which ion has the stronger HH bond? Surprisingly, the hybridization of the starred oxygen in the following molecule is sp 2, Molecular Orbital Theory Next. Molecular orbital theory is more powerful than valencebond theory because the orbitals reflect the geometry of the molecule to which they are applied. But this power carries a significant cost in terms of the ease with which the model can be visualized. In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. The central importance of the electron pair for bonding arises naturally in MO theory via the Pauli exclusion principle. A single electron pair is the maximum number that can occupy a bonding orbital and hence give the greatest lowering of energy. A molecular orbital (MO) can be used to represent the regions in a molecule where an electron occupying that orbital is likely to be found. Molecular orbitals are obtained from the combination of atomic orbitals, which predict the location of an electron in an atom..